Accelerate your protein engineering. Download our free guide to cell display
The Impact of Post-Translational Modifications in Mammalian Protein Production and Antibody Discovery
Post-translational modifications (PTMs) are a fundamental layer of biological regulation that dictates a protein's function and viability as a drug. This guide explores the most significant PTMs in mammalian production and antibody discovery, from glycosylation's role in stability and efficacy to the importance of correct disulfide bond formation. Understanding this complex landscape is essential for protein engineers to design effective biologics and avoid common developability risks that can compromise a therapeutic candidate.
10/7/20254 min read
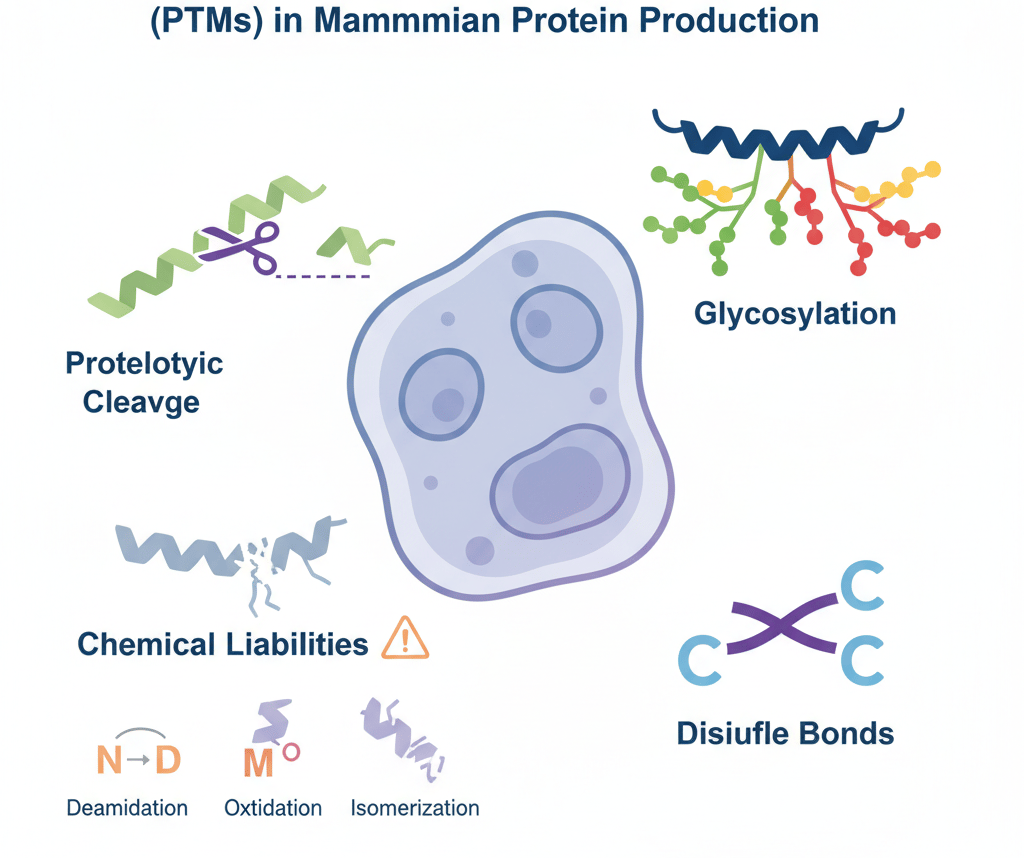
After a protein is synthesized by the ribosome, the job is not done. The protein still has to undergo a series of chemical alterations known as post-translational modifications (PTMs). These modifications are not simple decorations. They are a fundamental layer of biological regulation that dictates a protein's structure, localization, activity, and ultimate fate.
Nowhere is this more critical than in antibody development and complex biologic drugs development. While simpler hosts like E. coli can produce vast quantities of protein, they lack the machinery to perform human-like PTMs required by molecules like antibodies. This is why mammalian expression systems like Chinese Hamster Ovary (CHO) cells have become the undisputed workhorses for successful antibody development and the biopharmaceutical industry at large. Modern antibody engineering campaigns, which often begin with discovery platforms like yeast display or initial screening in mammalian display, must account for PTMs from the very beginning to ensure that a promising binder can become a viable therapeutic.
Understanding PTMs is essential for any protein engineer. These modifications can be the difference between a potent, stable therapeutic and a heterogeneous, ineffective, or even immunogenic product.
This following list of PTMs have provide profound impact on antibody and biologic drug quality.
Glycosylation: A Regulator of Function and Stability
Glycosylation—the enzymatic addition of sugar chains (glycans)—is arguably the most important and complex PTM for antibodies and biologic proteins. It directly influences a protein's folding, stability, serum half-life, and biological activity. The two primary types are:
N-linked Glycosylation: Occurs on the nitrogen atom of an asparagine (N) residue, typically within the sequon N-X-S/T (where X is any amino acid except proline). For antibody development, the conserved N-linked glycan in the Fc region is a regulator of its effector function. The specific composition of this glycan dictates the antibody's ability to engage with Fc receptors on immune cells.. A key consideration in antibody engineering is that the glycosylation pattern from a yeast display system will differ significantly from a final mammalian display or CHO production system, making early characterization critical.
O-linked Glycosylation: Occurs on the oxygen atom of serine (S) or threonine (T) residues. While less common in standard antibodies, it is a key feature of many other therapeutic proteins and fusion proteins.
While essential for function, glycosylation is also a major source of heterogeneity. The precise glycan structure can vary, creating a population of "glycoforms." Furthermore, unintended glycosylation at a newly created N-X-S/T site (e.g., in a CDR loop) is a major developability risk that can impair antigen binding.
Disulfide Bond Formation: The Architect of Structure
For complex molecules in antibody development, achieving the correct three-dimensional structure is paramount. This structure is locked in place by disulfide bonds, covalent links formed between the thiol groups of two cysteine (C) residues.
The Process: This oxidative folding process occurs within the endoplasmic reticulum (ER) and is guided by a host of cellular machinery, including enzymes like protein disulfide isomerase (PDI). For a typical IgG antibody, multiple cysteine residues must be correctly paired to create the iconic Y-shape, a process faithfully recapitulated in both yeast display and mammalian display systems.
The Impact: Incorrect disulfide bond pairing leads to misfolding, loss of function, and often triggers the cell's quality control machinery to degrade the protein. The presence of unpaired cysteines on a final product is a major developability red flag, as it can lead to the formation of aggregates.
Proteolytic Cleavage: Essential Maturation and a Source of Heterogeneity
Many proteins are synthesized as inactive precursors that require proteolytic cleavage to become mature and functional.
Signal Peptide Removal: The most universal example is the cleavage of the N-terminal signal peptide, which directs the nascent polypeptide chain into the secretory pathway. Incomplete cleavage can lead to a heterogeneous product with a different N-terminus.
C-terminal Clipping: For antibodies, clipping of the C-terminal lysine residue on the heavy chain is a common modification that can also contribute to product heterogeneity. While often having minimal impact on function, it must be monitored and controlled to ensure batch-to-batch consistency.
"Problematic" PTMs: The Chemical Liability Hotspots
Beyond the enzymatically controlled modifications, several spontaneous chemical degradation pathways can occur during production and storage. These are often considered undesirable PTMs and are flagged as developability risks.
Deamidation: The spontaneous conversion of an asparagine (N) residue into aspartic acid or isoaspartic acid. This introduces a charge variant and can alter a protein's structure and function. It is particularly common in flexible regions and at N-G (Asn-Gly) and N-S (Asn-Ser) motifs.
Oxidation: The modification of susceptible amino acid side chains, most commonly methionine (M) and tryptophan (W). Oxidation of a methionine residue within a CDR can dramatically reduce an antibody's binding affinity.
Isomerization: The conversion of aspartic acid (D) into its structural isomer, isoaspartate, which can disrupt the protein backbone and impact stability.
Conclusion: PTMs as a Critical Quality Attribute
Post-translational modifications are not an afterthought in antibody development and protein production; they are a central determinant of a biologic's safety, efficacy, and viability as a drug. The ability of mammalian cells to perform these complex modifications is the primary reason for their dominance in the industry. For those working in antibody engineering with platforms like yeast display and mammalian display, a deep understanding of this landscape is crucial. It informs everything from initial sequence design (e.g., avoiding liability hotspots) to the interpretation of analytical data. Ultimately, controlling and characterizing PTMs is a core challenge in modern antibody development, ensuring that we can consistently manufacture the high-quality, effective medicines that patients rely on.
Are you developing an antibody biologic?
Thinking about PTM from day one is key to success. Our Ph.D level team acts as a seamless extension of yours, providing the critical data you need to select and engineer candidates with the highest probabiliy of success.
Let's discuss how we can accelerate your next project
