Accelerate your protein engineering. Download our free guide to cell display
Library Size Limitations in Yeast Display: Advanced Strategies for Maximizing Diversity
Discover proven strategies to maximize yeast display library diversity despite transformation limitations. Learn advanced techniques including Golden Gate assembly, sequential enrichment, and smart library design that biotech and pharma companies use to achieve superior protein engineering results within practical constraints.
8/11/20257 min read
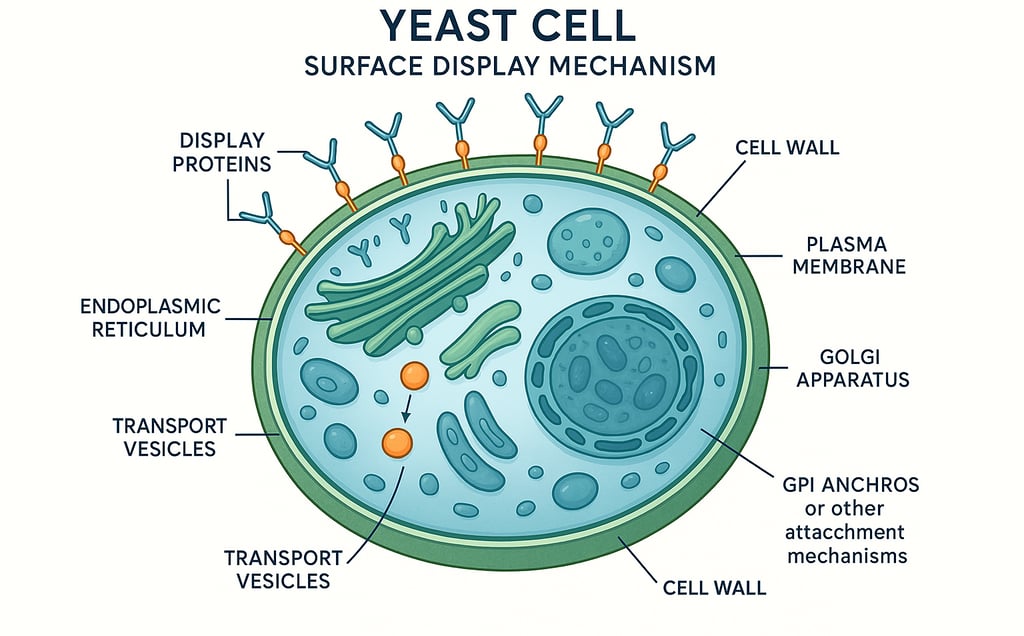
The success of any yeast display campaign fundamentally depends on the diversity and quality of the initial library. Yeast surface display offers advantages for protein engineering through its eukaryotic folding machinery and post-translational modifications. However, it faces library size limitations that can become a critical bottleneck for project outcomes. Understanding and addressing this challenge is essential for biotech and pharmaceutical companies seeking to maximize their return on investment in protein engineering campaigns.
The Library Size Challenge: Understanding the Fundamental Constraint
Yeast display libraries typically achieve diversities in the range of 10e7 to 10e9 unique variants, representing a significant constraint compared to phage display systems that routinely generate libraries containing 10e11 to 10e12 variants. This two to three order of magnitude reduction in theoretical diversity can heavily affect project success rates, particularly for projects requiring the identification of rare, high-affinity variants or proteins with highly specific functional properties.
The root cause of this limitation lies in the inherent constraints of yeast transformation efficiency. Unlike bacteriophages, which can achieve near-perfect infection rates through highly evolved mechanisms, yeast transformation relies on the uptake of plasmid DNA through chemically or electrochemically permeabilized cell walls. Even under optimal conditions, transformation efficiencies rarely exceed 10e6 to 10e7 transformants per microgram of DNA, and this efficiency decreases significantly with larger plasmids or more complex DNA constructs.
The mathematical implications become apparent when considering the relationship between library size and sequence space sampling. For antibody engineering applications, where researchers typically optimize both heavy and light chain variable regions simultaneously, the theoretical sequence space can exceed 10e20 possible combinations. With practical library sizes limited to 10e8 variants, researchers sample only a tiny fraction of available sequence space, significantly reducing the likelihood of identifying optimal variants.
This constraint is particularly problematic for pharmaceutical companies developing therapeutic antibodies, where the requirements for high affinity, specificity, stability, and favorable biophysical properties create a multi-dimensional optimization challenge. Smaller libraries are more susceptible to sampling bias, where certain regions of sequence space may be over- or under-represented due to stochastic effects during library construction. This bias can lead to the systematic exclusion of potentially valuable variants and may result in the identification of suboptimal solutions.
Library Construction Strategies
Optimized Transformation Protocols
The foundation of successful large-scale yeast library construction lies in optimizing transformation protocols to achieve maximum efficiency and reproducibility. The most effective approaches employ electrocompetent yeast cells prepared using specialized protocols that maximize cell wall permeability while maintaining cell viability. Critical factors include the growth phase at which cells are harvested, with mid-logarithmic phase cells generally providing optimal transformation efficiency, and the use of specific buffer compositions that enhance DNA uptake without compromising cell integrity.
Optimized electroporation protocols can routinely achieve transformation efficiencies exceeding 10e7 transformants per microgram of DNA. These protocols typically involve multiple rounds of optimization for specific yeast strains and plasmid systems, with careful attention to parameters such as voltage, capacitance, resistance, and pulse duration. The use of specialized electroporation cuvettes with optimized electrode spacing and the inclusion of specific additives in the electroporation buffer can further enhance transformation efficiency.
The preparation of electrocompetent cells requires meticulous attention to detail. Cells should be harvested during mid-logarithmic growth phase when cell wall permeability is optimal, typically at an OD600 of 0.6-0.8. The washing procedure must remove all traces of salts and media components that could interfere with electroporation, while maintaining cell viability through the use of appropriate osmotic stabilizers. Temperature control throughout the preparation process is also critical, as elevated temperatures can reduce transformation efficiency and cell viability.
Golden Gate Cloning for Enhanced Library Construction
Golden Gate cloning has emerged as a particularly powerful tool for constructing highly diversed libraries, offering several advantages over traditional cloning approaches. This method enables the simultaneous assembly of multiple DNA fragments in a single reaction, reducing the number of cloning steps required and minimizing the loss of diversity that can occur during sequential cloning procedures. The high efficiency and fidelity of Golden Gate assembly make it particularly well-suited for constructing combinatorial libraries where multiple variable regions must be assembled in different combinations.
The implementation of Golden Gate cloning for yeast display libraries requires careful design of the DNA fragments and assembly strategy. Optimal results are typically achieved when the variable regions are designed with compatible overhang sequences that enable efficient assembly while minimizing the formation of incorrect products. The use of type IIS restriction enzymes with different recognition sequences for each assembly junction helps ensure proper fragment orientation and reduces the likelihood of unwanted recombination events.
The design of Golden Gate assembly reactions must consider the stoichiometry of DNA fragments, enzyme concentrations, and reaction conditions. Typically, equimolar ratios of DNA fragments provide optimal assembly efficiency, although some optimization may be required for specific applications. The use of high-fidelity DNA polymerases and optimized buffer conditions can further improve assembly efficiency and reduce the formation of unwanted byproducts.
Sequential Enrichment Strategies
Sequential enrichment strategies allow for maximizing the effective diversity of yeast display libraries within transformation constraints. Rather than attempting to construct a single massive library, this approach involves the construction of multiple smaller libraries that are screened independently, with promising variants from each library combined for subsequent rounds of optimization. This strategy can be particularly effective when combined with computational design approaches that guide the selection of sequence regions to be diversified in each library.
The implementation of sequential enrichment requires careful planning of the diversification strategy and screening protocols. Each sub-library should target different regions of the protein or different types of mutations, ensuring comprehensive coverage of the relevant sequence space. The screening conditions for each sub-library may need to be optimized independently, as different types of mutations may require different selection pressures to identify optimal variants.
The combination of variants from different sub-libraries can be achieved through several approaches, including DNA shuffling, overlap extension PCR, or direct cloning of selected variants into new library constructs. The choice of combination method depends on the specific requirements of the project and the nature of the mutations being combined. Care must be taken to ensure that beneficial mutations from different sub-libraries are properly combined and that the resulting library maintains adequate diversity.
Smart Library Design Approaches
The concept of "smart" library design has gained increasing attention as a means of maximizing functional diversity within size-constrained libraries. Rather than employing completely random mutagenesis, these approaches use structural information, sequence analysis, and computational modeling to focus diversification efforts on regions most likely to yield functional improvements. By concentrating mutations in key binding regions or structural elements, researchers can achieve greater functional diversity with smaller library sizes compared to random approaches.
Computational tools for smart library design include structure-based design algorithms that identify residues likely to be important for binding or function, sequence analysis methods that identify conserved and variable regions in protein families, and machine learning approaches that predict the effects of specific mutations. These tools can be used individually or in combination to guide library design decisions and optimize the distribution of mutations within the library.
The implementation of smart library design requires access to structural information about the target protein and its binding partners, as well as computational expertise to analyze and interpret the results of design algorithms. While this approach requires more upfront investment compared to random mutagenesis, it can significantly improve the success rate of protein engineering campaigns and reduce the time and resources required to identify optimal variants.
Quality Control and Library Validation
Analytical Assessment of Library Composition
Quality control measures during library construction are particularly critical when working with size-constrained libraries. Effective quality control strategies include analytical assessment of library composition using next-generation sequencing, functional testing of library subsets to ensure proper protein expression and display, and negative control experiments to assess background binding and non-specific interactions.
Next-generation sequencing provides comprehensive information about library composition, including the distribution of mutations, the presence of unwanted sequences, and potential biases in library construction. This information can be used to identify problems with library construction protocols and guide optimization efforts. The analysis of sequencing data requires specialized bioinformatics tools and expertise, but the investment in proper analysis can prevent costly mistakes and improve library quality.
Functional testing of library subsets involves the random selection of individual clones from the library and assessment of their expression levels, display efficiency, and binding properties. This testing can identify problems with protein folding, expression, or display that may not be apparent from sequence analysis alone. The results of functional testing can guide decisions about library screening strategies and help optimize experimental conditions.
Expression and Display Monitoring
The monitoring of protein expression and display levels is essential for ensuring library quality and identifying potential problems with library construction or screening protocols. Flow cytometry-based methods can provide quantitative measurements of expression levels across library populations, enabling the identification of expression biases and the optimization of experimental conditions.
The use of dual-labeling approaches, where both protein expression and binding activity are measured simultaneously, can provide valuable information about the relationship between expression levels and functional activity. This information can be used to identify and correct for expression-level bias during screening and to optimize library construction protocols for improved expression uniformity.
Monitoring expression levels over time can also provide insights into library stability and the effects of culture conditions on protein expression and display. This information is particularly important for long-term screening campaigns or when libraries must be stored for extended periods before use.
Ranomics: Your Partner in Overcoming Library Size Challenges
For biotech and pharmaceutical companies facing the complexities of yeast display library construction, partnering with experienced specialists can significantly accelerate project timelines and improve success rates. Ranomics offers comprehensive yeast display services that address the library size challenge through extensive experience in optimizing library construction for diverse protein targets.
Our team has developed proprietary protocols for maximizing transformation efficiency and library diversity, incorporating the latest advances in cloning, sequential enrichment strategies, and smart library design. We understand that every protein engineering project has unique requirements, and we work closely with our biotech and pharma clients to develop customized library construction strategies that maximize the probability of success within budget and timeline constraints.
Ranomics' full-service approach means that clients can leverage our expertise throughout the entire protein engineering workflow, from initial library design and construction through screening, optimization, and final variant characterization. Our experience with both yeast display and mammalian display systems enables us to recommend the optimal platform for each specific application, ensuring that clients achieve the best possible results for their investment.
The library size challenge in yeast display is not insurmountable, but it requires careful planning, optimized protocols, and extensive experience to address effectively. By partnering with Ranomics, biotech and pharmaceutical companies can access the expertise and resources needed to overcome these challenges and achieve their protein engineering objectives efficiently and cost-effectively.
Our commitment to staying at the forefront of yeast display technology means that our clients benefit from the latest advances in library construction methods, quality control protocols, and screening strategies. Whether you're developing therapeutic antibodies, optimizing enzyme properties, or engineering novel binding proteins, Ranomics has the expertise and capabilities to help you succeed in your protein engineering endeavors.
