Accelerate your protein engineering. Download our free guide to cell display
Deconvoluting Polyclonal Hits: Strategies for Characterizing Enriched Library Pools
Your yeast display and mammalian display screen is finished, but now you face a complex set of NGS data where simply choosing the most abundant clone can lead to costly mistakes. This guide provides a strategic framework for deconvoluting polyclonal hits by moving beyond simple frequency to analyze enrichment ratios and patterns of convergent evolution. By applying these principles, you can confidently identify and select truly superior candidates, ensuring the success of your protein engineering campaign.
9/22/20254 min read
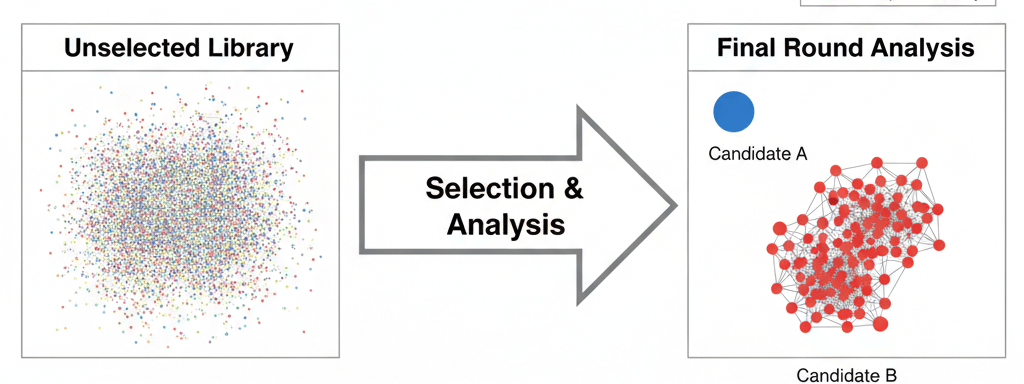
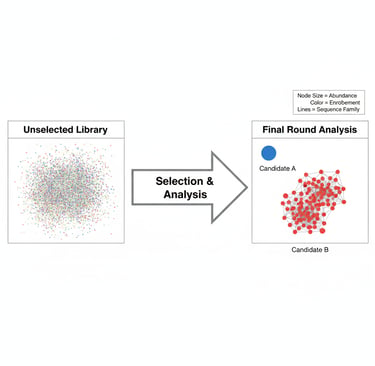
The final sort and sequencing cycle of a yeast or mammalian display campaign marks an important moment in any protein engineering project. After weeks of careful selection, you have successfully enriched a polyclonal population of yeast display or mammalian display cells, with each cell containing a unique sequence that may be the potential solution to your protein engineering challenge. In this final dataset, there will be a complex landscape of unique sequences, all of which performed well in the screen.
The success of the entire campaign now hinges on your ability to "deconvolute" this complex dataset. How do you look at a list of thousands of enriched variants and confidently select the top 5-10 candidates for downstream validation? The most common mistake is to simply rank the clones by their final abundance and pick the top hits. This approach is fraught with peril, as it overlooks the dynamics of selection and can lead to the selection of artifacts over truly superior variants.
Provided here is a strategic framework for interrogating polyclonal NGS data, moving beyond simple abundance to identify clones with the highest probability of functional success.
The Foundation: Why Simple Abundance is Not Enough
Relying solely on the final frequency of a clone is a flawed strategy because it ignores the history of the selection process. A clone can be highly abundant for reasons other than elite performance:
"Jackpot" Effect: A variant that was highly over-represented in the initial library (due to synthesis or cloning bias) may remain abundant throughout the screen without being a top performer.
PCR Amplification Bias: Some sequences are more easily amplified by PCR than others, which can artificially inflate their apparent numbers during NGS library preparation.
Modest Binders with High Display Levels: A cell displaying a million copies of a mediocre binder can be brighter—and thus more easily sorted—than a cell displaying a thousand copies of an elite binder, especially in early rounds.
The key is not to ask "Which clone is most common at the end?" but rather, "Which clone showed the most significant and consistent improvement throughout the selection?"
The Primary Metric: Calculating Enrichment Ratios
The most powerful quantitative tool for identifying high-performing variants is the enrichment ratio. This metric normalizes for a variant's starting frequency, revealing its performance relative to the rest of the population.
One simple way to think about this concept is the change in variant frequency over time. A simple calculation can be Enrichment Ratio = (Frequency of Variant in Final Round) / (Frequency of Variant in Unselected Library). A high performing variant would have increase in enrichment in each bind, sort and sequencing cycle.
To do this effectively, you must deep-sequence both your final enriched pool and your initial, unselected (Round 0) library. A variant that started at a frequency of 0.001% and ended at 1% (a 1000x enrichment) is often far more interesting than a variant that started at 0.5% and ended at 2% (a 4x enrichment). The enrichment ratio uncovers the hidden gems—the rare starting clones that dramatically outcompeted their peers.
Identifying Convergent Evolution: The Power of Sequence Families
Beyond individual enrichment ratios, the most compelling evidence for a successful solution comes from convergent evolution. This is the principle that a complex problem (like binding a specific epitope) will often be solved by the selection process in several similar, but not identical, ways. Instead of analyzing individual sequences in isolation, the next step is to cluster them into "sequence families" based on similarity.
This analysis reveals critical patterns:
Are entire families enriching? If a cluster of 20 related sequences all show high enrichment ratios, it provides immense confidence that this structural solution is robust and effective. It's a form of internal validation performed by the experiment itself.
Are there consensus mutations? By aligning the sequences within an enriching family, you can identify key consensus mutations—specific amino acid changes at certain positions that are clearly driving the improved function.
Are there shared motifs across different families? Sometimes, different sequence families will independently discover the same solution at a key position (e.g., a critical tyrosine in the CDR3 loop). This is one of the strongest possible indicators of a functionally essential mutation.
Putting It All Together: A Candidate Selection Framework
With these principles in mind, you can move from raw data to a short-list of top candidates. Instead of a simple ranked list, consider building a selection matrix or "scorecard" for your top families and individual clones, evaluating them on multiple criteria:
Enrichment Ratio: What is the quantitative measure of its success?
Final Abundance: Is the clone abundant enough to be considered real and not a sequencing artifact?
Family Convergence: Is this variant part of a larger, enriching family? How large is that family? This is your confidence score.
Sequence Liabilities: Does the sequence contain any red flags for downstream development, such as glycosylation sites, deamidation motifs, or unpaired cysteines?
Let's consider a hypothetical case:
Candidate A: The #1 most abundant clone (5% final frequency). It has a modest 15x enrichment ratio and is an "orphan"—no other similar sequences enriched alongside it.
Candidate B: The #30 most abundant clone (0.5% final frequency). It has a massive 800x enrichment ratio. Crucially, it is the lead member of a family of 25 other enriching variants, all of which share a key mutation at position H52.
Conclusion: Candidate B is a far more compelling and well-validated hit than Candidate A. The combination of a high enrichment ratio and strong family convergence gives you high confidence that its shared mutation is functionally critical.
Conclusion: From Data to Discovery
Deconvoluting a polyclonal NGS dataset is an investigative process that blends quantitative analysis with biological intuition. By moving beyond simple abundance and focusing on enrichment ratios and patterns of convergent evolution, you can dramatically increase the probability of selecting truly exceptional candidates. This rigorous, multi-faceted approach ensures that the immense effort invested in a display campaign is translated into the successful discovery of high-performing, well-validated biologics.
De-Risk Your Biologic Development Pipeline
Are you working on a pooled screening campaign and hitting roadblocks?
Our Ph.D.-level team acts as a seamless extension of yours, providing the critical data you need to select and engineer candidates with the highest probability of success.
Let's discuss how we can accelerate your next project.
