Accelerate your protein engineering. Download our free guide to cell display
Correctly Titrating Display Levels for Reliable Affinity Data in Yeast & Mammalian Systems
Learn how to optimize display-level titration in yeast and mammalian display systems to obtain accurate affinity data and avoid avidity effects when determine Kd using yeast and mammalian display
8/28/20258 min read
The Affinity Data Dilemma
Let's start with a scenario many researchers in protein engineering face. You've spent weeks optimizing your yeast or mammalian surface display experiment. Your flow cytometry data looks clean, the resulting binding curve is a perfect sigmoid, and you calculate an impressive, low-nanomolar Kd. But when you repeat the experiment, the value shifts significantly. Or worse, a downstream biophysical measurement doesn't match your display data at all. Sound familiar?
This frustrating inconsistency often stems from a hidden artifact that can invalidate otherwise perfect-looking data: avidity. Without carefully controlled experimental conditions, it's remarkably easy to measure the combined strength of multi-site binding (avidity) rather than the true, 1:1 intrinsic affinity you're actually searching for.
The key to avoiding this common pitfall lies in a critical, yet often overlooked, step: correctly titrating your display levels. This guide provides al walkthrough for establishing reproducible binding curves in both yeast and mammalian display systems, ensuring your affinity data is not just beautiful, but truly reliable and reproducible.
The Core Principle: Why Display Level is Critical
To understand why titration is so important, we first need to distinguish between two fundamental concepts: affinity and avidity. While they are often used interchangeably in casual conversation, in the context of a binding experiment, they are critically different.
Affinity (KD) describes the intrinsic binding strength between a single binding site of your displayed protein and a single epitope of its target antigen. This is a monovalent interaction, and it's almost always the value we want to measure.
Avidity describes the overall accumulated strength of multiple simultaneous interactions. This occurs when a high density of proteins on the cell surface collectively binds to the antigen. Think of it like Velcro®: a single hook-and-loop pair is weak (affinity), but the combined strength of thousands of pairs is immense (avidity).
In a surface display experiment, if the expression level of your protein is too high, you create a dense field of binding sites. This makes it highly probable that your labeled antigen will be bound by multiple displayed proteins simultaneously, or recaptured quickly after dissociation. This avidity effect can dramatically and artificially lower your apparent Kd, making a weak binder look like a potent one.
The Goal: The Stoichiometric Binding Regime
This brings us to the central goal of our titration: to find the stoichiometric (or "monovalent") binding regime.
This is the experimental sweet spot where the surface density of your displayed protein is low enough that, on average, each labeled antigen molecule can only interact with a single displayed protein at a time. When you operate within this 1:1 regime, the measured fluorescence signal is directly proportional to the true binding affinity.
The entire purpose of the titration methods we'll discuss next is to verify that you are operating within this 1:1 binding regime before you even begin your full affinity measurement experiment.
Step-by-Step Guide: Titration in Yeast Display
Yeast display is a powerful and robust system, and verifying your binding regime is straightforward. The most common method involves creating a gradient of protein expression and checking if antigen binding scales linearly with the amount of protein on the surface.
1. Control Your Expression Level
The goal is to generate several yeast populations, each with a different average level of protein display. If you're using the common galactose-inducible GAL1 promoter, the easiest way to do this is with a time course.
Induction: After growing your yeast in a non-inducing and selection medium (i.e. Raffinose/-Trp), transfer them to an inducing medium (i.e. Galactose/-Trp)
Time Points: Pull out aliquots of your culture at different time points. For example: 2, 4, 8, and 16 hours of induction at a lower temperature like 20°C. Lower temperatures often lead to better protein folding and more uniform display. Keep an uninduced sample as a negative control.
2. Set Up the Labeling Experiment
For this experiment, you'll use a single, fixed concentration of your labeled antigen.
Choosing an Antigen Concentration: A good starting point is a concentration near your expected KD. If the affinity is unknown, try something in the low-nanomolar range (e.g., 10 nM). Remember, the goal here isn't to measure the KD, but to check for linearity.
Co-labeling: You must co-label your yeast with your fluorescently-tagged antigen (e.g., conjugated to Alexa Fluor 647) AND a fluorescently-tagged antibody against your expression tag (e.g., anti-c-myc antibody conjugated to PE). This allows you to measure both antigen binding and protein expression simultaneously.
Incubation: Incubate your different induced yeast populations with this labeling master mix, wash, and prepare for flow cytometry.
3. Flow Cytometry & Gating
After gating on your main yeast population and then on single cells (singlets), the key analysis is on a 2D plot:
X-axis: Expression Level (e.g., anti-c-myc PE signal)
Y-axis: Antigen Binding (e.g., Antigen-AF647 signal)
As you look at your samples from different induction time points, you should see the population "cloud" move up and to the right on this plot, indicating that longer induction leads to more expression and more antigen binding.
4. Data Analysis & Interpretation
This is where you get your answer.
Extract MFIs: For each induction time point, calculate the Mean Fluorescence Intensity (MFI) for both the expression tag channel and the antigen channel.
Plot MFI vs. MFI: Create a new graph plotting Antigen MFI (Y-axis) against Expression Tag MFI (X-axis).
The result you want to see is a straight line. A linear relationship is the definitive proof that antigen binding is directly proportional to the amount of displayed protein. This confirms you are in the monovalent, 1:1 binding regime.
If the line begins to curve and plateau at the higher expression levels, it's a red flag.. This indicates that binding is saturating due to avidity effects. For proper experimentation, choose an induction timepoint that falls on the linear part of the MFI vs MFI blot, this will ensure that avidity effects will not affect downstream reproducibility. This induction time point can also be used for a titration of the antigen, which will provide a reliable Kd value.
Step-by-Step Guide: Titration in Mammalian Display
Working with mammalian cells, like HEK293 or ExpiCHO, requires a slightly different approach primarily due to expression heterogeneity. Unlike a relatively uniform induced yeast culture, a transiently transfected pool of mammalian cells will contain a full spectrum of cells expressing your protein at low, medium, and very high levels, all within the same tube.
While this sounds like a problem, we can actually leverage this heterogeneity to our advantage.
1. Unique Challenges & Strategy
The main challenge is the lack of uniform expression. The solution is to use the expression gradient within a single sample to perform your entire titration analysis. Instead of creating different populations over time, you will analyze the relationship between expression and binding across the single, diverse population.
It is absolutely critical to include a viability dye (e.g., DAPI, PI, or Zombie Dyes) in your staining panel. Dead cells non-specifically bind proteins and will ruin your data, so your first gate must always be on the "Live" cell population.
2. Experimental Setup & Gating
The setup is similar to the yeast workflow, but your analysis will be done on one or two key samples.
Transfect Your Cells: Perform your transient transfection as you normally would.
Labeling: At your desired timepoint post-transfection (e.g., 24-48 hours), harvest the cells. As with yeast, label them with a single, fixed concentration of your fluorescently-tagged antigen and a fluorescently-tagged antibody against your expression tag (e.g., anti-FLAG or anti-HA). Crucially, also add your viability dye.
Gating Strategy: Your gating hierarchy is paramount.
First, gate on Live Cells (e.g., DAPI-negative).
Next, gate on Singlets to exclude cell clumps.
From this clean, live, single-cell population, you will analyze expression and binding.
3. Data Analysis: The Single-Sample Power Plot
This is where the magic happens. For your clean, live, single-cell population, generate a 2D plot of antigen binding vs. expression level.
X-axis: Expression Level (e.g., anti-FLAG PE signal)
Y-axis: Antigen Binding (e.g., Antigen-AF647 signal)
Because your single sample contains cells with a wide range of expression levels, you will see a diagonal "smear" of events starting from the double-negative population and extending up and to the right.
The result you want to see is a straight, linear relationship. This line of cells on your plot demonstrates that even as the expression level increases, the antigen binding signal increases proportionally. This is your proof of a 1:1 stoichiometric binding regime.
If the line of cells begins to bend horizontally (plateau) at the high-expression end, it's an indication for avidity. The highest-expressing cells are no longer in the 1:1 regime.
You now know the "safe zone." For your full dose-response experiments to determine the Kd, you can apply a gate on this exact plot to include only the cells that fall within the linear range and exclude the high-expressing, plateaued cells from your analysis. This simple gating strategy ensures your final affinity data is derived only from cells giving a true affinity-based signal.
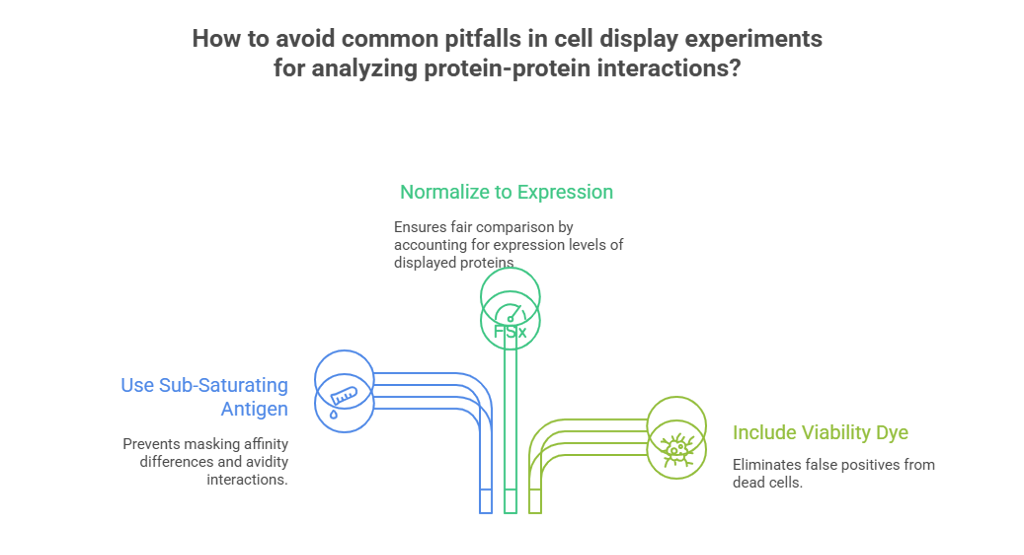
Common Pitfalls and How to Avoid Them
You've followed the protocol, but your data still looks strange. Before you start from scratch, check if you've fallen into one of these common traps. Getting this part right is just as important as the protocol itself.
1. Pitfall: Using Too Much Antigen
This is the single most common error. In an attempt to get a bright, clean signal, it's tempting to use a high, saturating concentration of your labeled antigen.
Why it's a mistake: A very high antigen concentration can mask subtle affinity differences and, more importantly, can force avidity interactions even on cells with lower display levels. It essentially makes everything look like a strong binder and defeats the purpose of your careful expression titration.
The Fix: For your initial experiments in determining avidity effects, always use a single, sub-saturating concentration of antigen (ideally at or below the expected KD). For your final KD measurement, you will use a range of concentrations, but ensure they span well below and above the expected KD.
2. Pitfall: Not Normalizing to Expression Level
It can seem simpler to just look at the antigen MFI for a population and draw a conclusion. This is almost always misleading.
Why it's a mistake: A sample might show a high antigen MFI simply because the cells are expressing a massive amount of a low-affinity protein. Conversely, a low overall MFI might hide a very potent binder that is just poorly expressed. The raw antigen signal, in isolation, is uninterpretable.
The Fix: This is precisely why you co-label with an anti-expression tag antibody. Always analyze your data on the "Antigen MFI vs. Expression MFI" plot. It normalizes binding to expression and reveals the true relationship, allowing you to compare binders fairly.
3. Pitfall: Ignoring Cell Viability
This is especially critical for mammalian systems, which are more fragile than yeast.
Why it's a mistake: Dead and dying cells have compromised membranes that become notoriously "sticky." They will non-specifically bind to your antibodies and antigens, creating a major source of false-positive signal that can dramatically skew your results.
The Fix: This is non-negotiable. Always include a viability dye (like DAPI, PI, or a fixable viability stain) in your mammalian staining panel. Your very first gate during flow cytometry analysis must be to analyze only the live, healthy cells.
Conclusion: Confidence in Your Curves
Generating an accurate KD value is more than just fitting a curve to a set of data points. It's about ensuring that the data itself reflects the true molecular interaction you're trying to measure. The difference between a beautiful-looking curve and a truly reliable one often comes down to one critical step: validating your binding regime.
As we've covered, the key is to first prove that your assay is operating in a stoichiometric, 1:1 environment where avidity isn't skewing your results. Whether you're using an induction time-course in yeast or leveraging the inherent expression heterogeneity in mammalian cells, the "Antigen MFI vs. Expression MFI" plot is your definitive test.
By taking the time to perform these titration controls, you move beyond simply generating data to producing robust, reproducible affinity measurements—data that you can trust, build upon, and publish with confidence.
Are you looking to start up a yeast display or mammalian display experiment and have questions?
Feel free to contact our team of experts anytime
