Accelerate your protein engineering. Download our free guide to cell display
Beyond FACS: An Introduction to Magnetic-Activated Cell Sorting (MACS) for Library Pre-enrichment
While FACS is the gold standard for precision sorting, it becomes a bottleneck when screening libraries with billions of variants. Magnetic-Activated Cell Sorting (MACS) offers a powerful solution by using magnetic microbeads to rapidly pre-enrich binders from massive populations. This guide introduces the MACS workflow and compares its strengths, like immense throughput, against the precision of FACS. The most effective screening campaigns use a hybrid approach, leveraging MACS for initial bulk separation followed by FACS for fine-tuned selection. By integrating MACS, researchers can accelerate discovery timelines and more efficiently tackle larger, more diverse libraries.
9/16/20253 min read
Fluorescence-Activated Cell Sorting (FACS) is the undisputed gold standard for precision sorting in surface display campaigns. Its ability to perform quantitative, multi-parameter analysis on single cells is unmatched for isolating elite candidates in later rounds of selection. However, when faced with a naive library of billions of variants, FACS can become a significant bottleneck due to limitations in speed, cost, and the sheer volume of cells to be processed.
For these initial, large-scale rounds, a complementary technology offers a powerful solution: Magnetic-Activated Cell Sorting (MACS). This guide introduces MACS as a high-throughput pre-enrichment tool that can dramatically improve the efficiency of your display campaigns by rapidly culling non-binders from massive libraries.
How MACS Works: From Fluorescence to Ferromagnetism
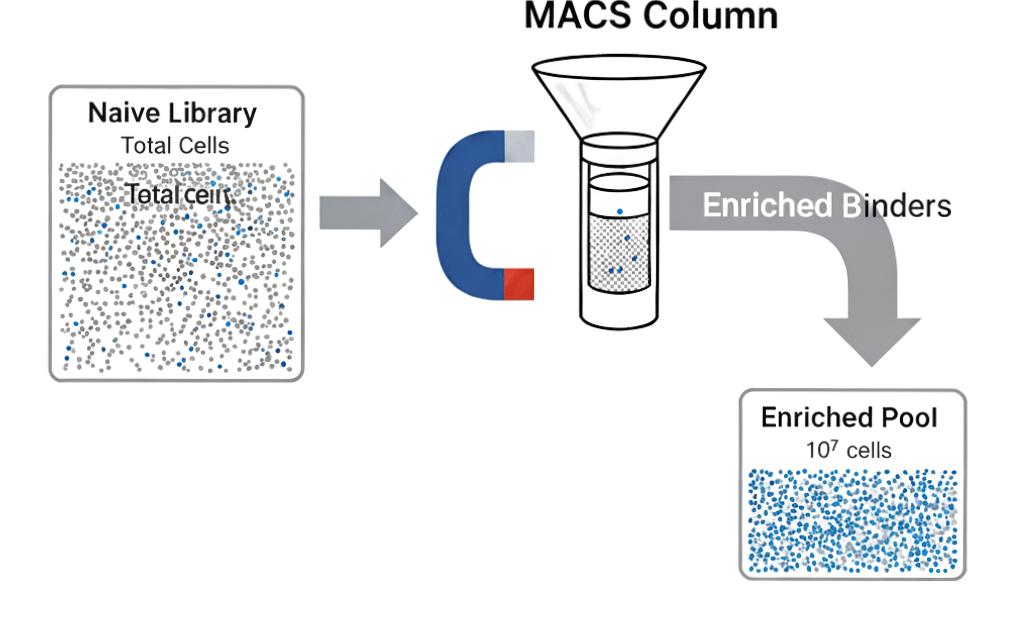
The principle behind MACS is simple and robust. Instead of labeling a target with a fluorophore, it is labeled with a superparamagnetic microbead. The workflow is straightforward:
Labeling: The display library (e.g., yeast) is incubated with a biotinylated target antigen. Only cells displaying a binding variant will become coated with the target.
Tagging: Streptavidin-conjugated magnetic microbeads (typically 50-100 nm in diameter) are added. These beads bind with high affinity to the biotinylated antigen, magnetically tagging the cells of interest.
Separation: The entire library is passed through a separation column placed within a powerful permanent magnet.
Enrichment: Magnetically tagged cells are retained in the column, while the vast majority of non-binding cells flow through and are discarded. The magnet is then removed, and the enriched population of binders is eluted.
This entire process can be completed in under an hour, requires minimal specialized equipment, and is exceptionally gentle on the cells.
The Strategic Role of MACS: Solving the "Needle in a Haystack" Problem
The primary advantage of MACS is its immense throughput. A standard high-speed cell sorter can process tens of millions of cells per hour. In contrast, MACS can process tens of billions of cells in the same timeframe. This makes it the ideal tool for the first round of selection against a large and diverse library, where the frequency of positive hits is extremely low.
Attempting to screen a 10e10 variant library with FACS would be impractical. With MACS, this initial "de-bulking" step is fast and efficient. It acts as a powerful filter, rapidly reducing the library's complexity by several orders of magnitude and enriching the population for a much smaller, more manageable pool of potential binders.
MACS vs. FACS: A Comparison of Strengths
MACS is not a replacement for FACS; it is a complementary tool with a different set of strengths.
Throughput: MACS has a significantly higher throughput (>10⁹ cells/hr) than FACS (~10⁷-10⁸ cells/hr), making it superior for initial bulk enrichment.
Precision: FACS offers unparalleled precision. It allows for quantitative measurement of fluorescence and multi-parameter gating (e.g., normalizing binding to expression), which MACS cannot do.
Purity: Because of its precision, a FACS sort yields a much higher purity of true positive hits. MACS is an enrichment tool, and the resulting population will still contain a background of low-affinity and non-specific binders.
Cell Stress: MACS is very gentle, as cells are not subjected to high pressure, shear forces, or laser interrogation. This can improve the viability of the enriched population.
The Hybrid Workflow: Combining Speed and Precision
The most effective screening campaigns leverage the strengths of both technologies in a hybrid workflow.
Round 1 (MACS): The naive library (10e9-10e10 cells) is subjected to MACS selection. This rapidly eliminates >99.9% of the non-binding clones, yielding an enriched pool of ~10⁶-10⁷ cells.
Rounds 2+ (FACS): This smaller, enriched population is then used for all subsequent rounds of sorting on a FACS machine. At this stage, the precision of FACS is critical for performing the fine-grained selections needed for affinity maturation, such as titrating antigen concentration or normalizing to expression.
This hybrid approach provides the best of both worlds: the raw speed of MACS for initial enrichment, followed by the quantitative precision of FACS for isolating elite candidates.
Conclusion: An Essential Tool for Large-Scale Screening
By integrating MACS as a pre-enrichment step, researchers can overcome the throughput limitations of FACS and efficiently tackle larger and more diverse libraries. This strategy not only accelerates the discovery timeline but also improves the overall quality of the screening campaign by ensuring that rare but valuable clones are not lost in the noise of a massive starting population.
