Accelerate your protein engineering. Download our free guide to cell display
Beyond Antibodies: Using Surface Display to Engineer Enzymes and Receptors
While surface display is the go-to platform for antibody discovery, applications extend far beyond. This technical guide explores the creative strategies required to engineer complex proteins like enzymes and receptors. Learn how to translate catalytic activity and receptor function into a sortable fluorescent signal using methods like fluorogenic substrates, product capture, and downstream signaling assays. Move beyond simple binding and unlock the full power of surface display for your next protein engineering project.
9/10/20253 min read
When protein engineers think of surface display, they almost invariably think of antibody engineering. For decades, yeast and mammalian display have been the workhorses for affinity maturation and the discovery of novel binders. But to limit these powerful platforms to antibodies is to overlook the fundamental theory that any protein of interest can be displayed on cell surfaces. This includes enzymes and receptors.
Surface display's core strength is its direct, physical linkage of genotype (the plasmid DNA inside the cell) to phenotype (the protein functioning on the cell surface). This enables the high-throughput interrogation of millions of variants using fluorescence-activated cell sorting (FACS). While screening for binding is straightforward, screening for catalysis or receptor function requires a creative leap. This guide explores the unique strategies required to adapt surface display for these complex applications.
Engineering Enzymes: Screening for Catalysis, Not Just Binding
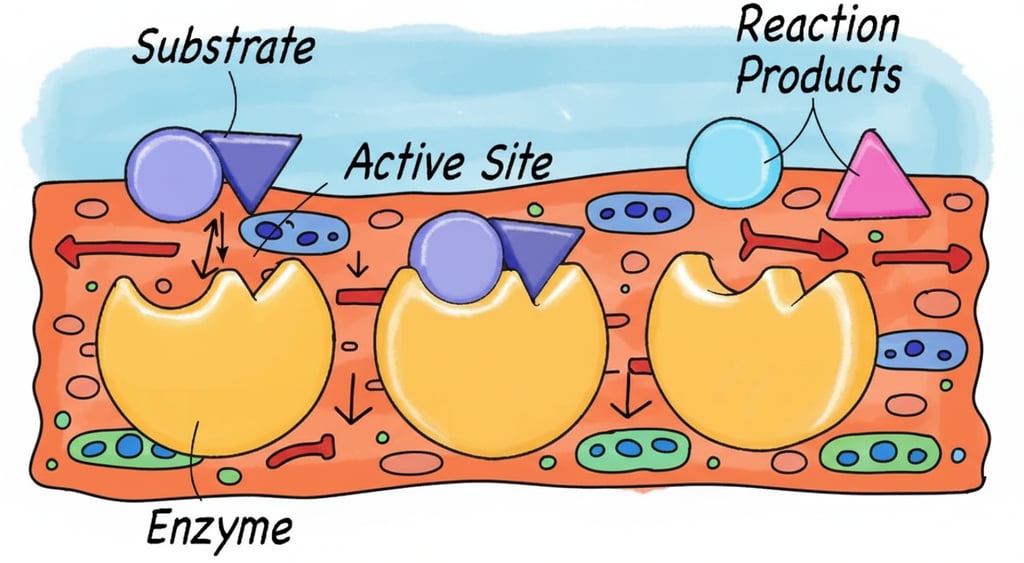
The fundamental challenge in enzyme engineering is measuring a dynamic catalytic reaction within the static snapshot of a FACS experiment. One strategy is to convert enzymatic activity into a stable fluorescent signal on the cell surface. This is achieved by designing an assay where the enzyme’s action generates a product that is retained on or near the cell.
The Challenge: From Catalysis to Fluorescence
Unlike a simple binding event, an enzymatic reaction must occur within a specific time window under conditions compatible with cell viability. The key challenge is ensuring the product is captured or detected before it diffuses away. Several clever strategies make this possible.
Strategies for Capturing the Signal at the Cell Surface
To successfully sort for enzymatic activity, the fluorescent signal must be localized to the cell that generated it. Here are several effective strategies to achieve this:
1. Leveraging Intrinsic Product Properties: The simplest method relies on the inherent chemical properties of the reaction product. If an enzyme cleaves a substrate to reveal a highly charged or hydrophobic moiety, the product may naturally associate with the cell surface. For example, a product with a strong positive charge will stick to the negatively charged yeast cell wall, localizing the signal. This is often used with fluorogenic substrates that become fluorescent upon conversion.
2. Biotin-Streptavidin Capture: This is one of the most robust and widely used methods. The substrate is synthesized with a biotin tag. The enzyme on the cell surface converts the substrate into a product, which remains localized (often due to intrinsic properties, as above). Then, fluorescently-labeled streptavidin is added. Streptavidin binds with incredibly high affinity to the biotin tag on the product, effectively "painting" the active cells with a bright, stable fluorescent signal.
3. Substrate Tethering: For the most direct linkage, the substrate itself can be chemically cross-linked to the cell surface. In this setup, the enzyme on the cell surface acts on a substrate that is already physically attached to its own cell. When the substrate is converted to a fluorescent product, the signal is inherently and covalently retained, providing a very clean and direct readout of activity.
4. Droplet Encapsulation: In a more advanced, high-throughput approach, cells from the library are encapsulated into millions of tiny water-in-oil -in-buffer droplets using microfluidics. Each droplet acts as a microscopic test tube containing a single cell and the reaction components (e.g., a fluorogenic substrate). The enzymatic reaction proceeds within the droplet, so the fluorescent product accumulates and is contained with the cell that produced it. The droplets themselves can then be sorted using a FACS machine.
Key Consideration for Enzymes: The kinetics of the reaction are paramount. You must optimize substrate concentration and incubation time to ensure the reaction proceeds far enough to distinguish active clones from inactive ones, without reaching saturation where all active clones appear equally bright.
Engineering Receptors: Screening for Function Beyond Affinity
Receptor engineering often goes beyond simple binding affinity. The goal might be to enhance stability, alter specificity, or modulate downstream signaling (agonism vs. antagonism). Surface display can be adapted to screen for all of these properties.
The Challenge: Linking Function to a Sortable Signal
Affinity and Specificity: This is the most straightforward application and is analogous to antibody screening. To improve affinity, cells are incubated with decreasing concentrations of a fluorescently-labeled ligand across sorting rounds. To improve specificity, a counter-screening strategy is essential. Cells are co-incubated with a "positive" target ligand (e.g., labeled with a red fluorophore) and a "negative" off-target ligand (labeled with a green fluorophore). The goal is to sort the cells that are red-positive but green-negative.
Enhanced Stability and Expression: A common goal for therapeutic receptors (like soluble decoy receptors) is improving stability and expression. Surface display provides an excellent proxy for this. Since only properly folded and stable proteins are trafficked efficiently to the cell surface, one can simply sort for the brightest cells using a fluorescent antibody against the receptor's expression tag (e.g., c-myc or HA tag). This enriches for variants with improved folding and expression characteristics. This can be combined with a stability challenge, such as a mild heat or pH treatment prior to staining.
Downstream Signaling: The most advanced application involves converting a receptor's signaling cascade into a fluorescent signal. This requires engineering the host cell itself. For example, a GPCR library can be expressed in a cell line that contains a downstream reporter gene, such as GFP.
