Accelerate your protein engineering. Download our free guide to cell display
A Technical Guide to Directed Evolution for Enhancing Protein Stability and Function
6/30/202527 min read


Directed evolution has matured from a novel academic concept into a transformative protein engineering technology, representing a paradigm shift in how new biological functions are created and optimized.1 It is a powerful, forward-engineering process that harnesses the principles of Darwinian evolution—iterative cycles of genetic diversification and selection—within a laboratory setting to tailor proteins for specific, human-defined applications.3 The profound impact of this approach was formally recognized with the 2018 Nobel Prize in Chemistry, awarded to Frances H. Arnold for her pioneering work that established directed evolution as a cornerstone of modern biotechnology and industrial biocatalysis.5
The primary strategic advantage of directed evolution lies in its capacity to deliver robust solutions—such as enhanced stability, novel catalytic activity, or altered substrate specificity—without requiring detailed a priori knowledge of a protein's three-dimensional structure or its catalytic mechanism.7 This capability allows it to bypass the inherent limitations of rational design, which relies on a predictive understanding of sequence-structure-function relationships that is often incomplete.3 By exploring vast sequence landscapes through a process of mutation and functional screening, directed evolution frequently uncovers non-intuitive and highly effective solutions that would not have been predicted by computational models or human intuition.9
Today, this technology is routinely deployed across the pharmaceutical, chemical, and agricultural industries to create enzymes and proteins with properties optimized for performance, stability, and cost-effectiveness.7 Applications range from developing highly stable enzymes for detergents and biofuel production to engineering therapeutic antibodies and viral vectors for gene therapy.10 This report provides Research and Development leaders with a comprehensive technical framework for understanding the methodologies, applications, and strategic considerations for deploying directed evolution. It offers a detailed examination of the techniques used to enhance protein stability and function, supported by landmark case studies, to inform strategic decision-making and resource allocation in the pursuit of next-generation biological products.
II. The Engine of Innovation: The Directed Evolution Cycle
At its core, directed evolution functions as a two-part iterative engine, relentlessly driving a protein population toward a desired functional goal.2 This process compresses geological timescales of natural evolution into weeks or months by intentionally accelerating the rate of mutation and applying an unambiguous, user-defined selection pressure.3 The iterative cycle consists of two fundamental steps: first, the generation of genetic diversity to create a library of protein variants, and second, the application of a high-throughput screen or selection to identify the rare variants exhibiting improvement in the desired trait.3 The genes encoding these "winners" are then isolated and used as the starting material for the next round of evolution, allowing beneficial mutations to accumulate over successive generations.4 A critical distinction from natural evolution is that the selection pressure is decoupled from organismal fitness; the sole objective is the optimization of a single, specific protein property defined by the experimenter.3
A. Principles of Laboratory-Accelerated Evolution
The directed evolution workflow is a powerful algorithm for navigating the immense and complex fitness landscapes that map protein sequence to function.4 A typical experiment begins with a single parent gene encoding a protein that possesses a basal level of the desired activity.4 This gene is subjected to mutagenesis to create a large and diverse library of variants.4 These variants are then expressed as proteins, and this population is challenged with a screen or selection that identifies individuals with improved performance.4 For example, to improve an enzyme's thermostability, the library might be heated to a temperature that denatures the parent protein, after which variants are screened for remaining catalytic activity.14 The genes from the most stable variants are then isolated, often recombined to bring together different beneficial mutations, and subjected to another round of mutagenesis and screening at an even higher temperature.4 This iterative process is repeated until the desired performance target is met or no further improvements can be found.4 The success of any directed evolution campaign hinges on the quality of the initial library and, most critically, the power of the screening method used to find the needle of improvement in the haystack of neutral or deleterious mutations.15
B. Step 1: Generating Genetic Diversity - The Library as the Search Space
The creation of a diverse library of gene variants is the foundational step that defines the boundaries of the explorable sequence space.3 The quality, size, and nature of this diversity directly constrain the potential outcomes of the entire evolutionary campaign.15 Several methods have been developed to introduce genetic variation, each with distinct advantages, limitations, and inherent biases that shape the evolutionary trajectories available to the protein.3
Random Mutagenesis Techniques
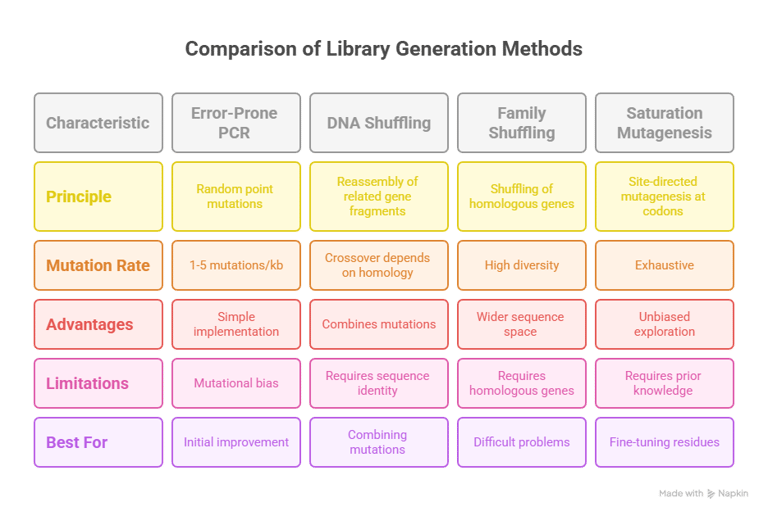
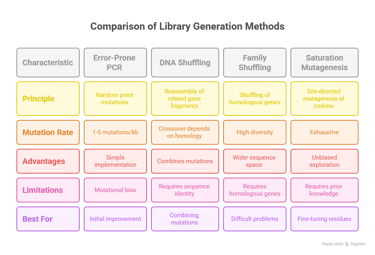
Random mutagenesis aims to introduce mutations across the entire length of a gene without pre-selecting specific sites.17 The most established and widely used method is Error-Prone Polymerase Chain Reaction (epPCR).7 This technique is a modified PCR that intentionally reduces the fidelity of the DNA polymerase, thereby introducing errors during gene amplification.18 This is typically achieved through a combination of factors: using a polymerase that lacks a 3' to 5' proofreading exonuclease activity (such as Taq polymerase), creating an imbalance in the concentrations of the four deoxynucleotide triphosphates (dNTPs), and, most critically, adding manganese ions (Mn2+) to the reaction.20 The concentration of Mn2+ can be precisely controlled to tune the mutation rate, which is typically targeted to 1–5 base mutations per kilobase, resulting in an average of one or two amino acid substitutions per protein variant.7
While powerful and straightforward, epPCR is not truly random.7 DNA polymerases have an intrinsic bias that favors transition mutations (purine-to-purine or pyrimidine-to-pyrimidine) over transversion mutations (purine-to-pyrimidine or vice versa).7 This bias, combined with the degeneracy of the genetic code, means that at any given amino acid position, epPCR can only access an average of 5–6 of the 19 possible alternative amino acids.7 This inherent limitation constrains the accessible sequence space and may prevent the discovery of an optimal variant if it requires a specific transversion mutation.7
Recombination-Based Methods (Gene Shuffling)
To overcome the limitations of point mutagenesis and to more closely mimic the power of natural sexual recombination, methods based on gene shuffling were developed.7 These techniques allow for the combination of beneficial mutations from multiple parent genes into a single, improved offspring.21
DNA Shuffling, also known as "sexual PCR," was pioneered by Willem P. C. Stemmer.7 In this method, one or more related parent genes are randomly fragmented using the enzyme DNaseI.7 These small fragments (typically 100–300 bp) are then reassembled in a PCR reaction without any added primers.7 During the annealing step, homologous fragments from different parental templates can overlap and prime each other for extension by the polymerase.7 This template switching results in crossovers, effectively shuffling the genetic information and creating a library of chimeric genes that contain novel combinations of mutations from the parent pool.7
A highly effective extension of this concept is Family Shuffling.7 This method applies the DNA shuffling protocol to a set of homologous genes isolated from different species.7 By drawing from the standing variation that nature has already created, family shuffling provides access to a much broader and more functionally relevant region of sequence space than mutating a single gene.7 It has been shown to significantly accelerate the rate of functional improvement compared to epPCR or single-gene DNA shuffling.7
The primary limitation of recombination-based methods is their requirement for sequence homology.7 The parental genes must typically share at least 70–75% sequence identity to ensure efficient and correct reassembly; with lower homology, the reaction strongly favors the regeneration of the original parent sequences.7 Furthermore, crossovers are not uniformly distributed and tend to occur more frequently in regions of high sequence identity, which can restrict the diversity of the resulting library.
Focused and Semi-Rational Mutagenesis
As an alternative to random approaches, focused mutagenesis targets specific regions or residues within a protein.23 This is often employed when some structural or functional information is available, allowing for the creation of smaller, higher-quality libraries.23
Site-Saturation Mutagenesis is a powerful example of this strategy.24 This technique is used to comprehensively explore the functional importance of one or a few amino acid positions, often "hotspots" identified from a prior round of random mutagenesis or predicted from a structural model.26 At the target codon, a library is created that encodes for all 19 other possible amino acids.26 This allows for a deep, unbiased interrogation of a residue's role, something that is statistically improbable with epPCR.24 This semi-rational approach, which combines knowledge-based targeting with random diversification at those sites, can dramatically increase the efficiency of a directed evolution campaign by reducing the library size and increasing the frequency of beneficial variants.23
The choice of diversification strategy is not a trivial decision; it is a strategic choice that shapes the entire evolutionary search.3 Relying on a single method can lead an experiment into an evolutionary dead end due to inherent methodological biases.7 For example, a campaign relying solely on epPCR may never find an optimal solution that requires a specific transversion mutation.7 A more robust R&D strategy often involves using a combination of methods sequentially.24 An initial round of epPCR might identify several beneficial mutations, which can then be combined using DNA shuffling.24 Finally, saturation mutagenesis can be used to exhaustively explore the key hotspots identified in the first two stages, ensuring the most thorough exploration of the most promising regions of the fitness landscape.24
C. Step 2: Linking Genotype to Phenotype - High-Throughput Screening and Selection
Once a diverse library of gene variants is created, the central challenge of directed evolution emerges: identifying the rare variants with improved properties from a population dominated by neutral or non-functional mutants.3 This step, which links the genetic code of a variant (genotype) to its functional performance (phenotype), is widely recognized as the primary bottleneck in the process.28 The success of a campaign is dictated by the axiom, "you get what you screen for".30 The power and throughput of the screening platform must match the size and complexity of the library generated in the first step.27
A key distinction exists between screening and selection.27 Screening involves the individual evaluation of every member of the library for the desired property.27 In contrast, selection establishes a system where the desired function is directly coupled to the survival or replication of the host organism, automatically eliminating non-functional variants.27 Selections can handle much larger libraries and are less labor-intensive, but they are often difficult to design, can be prone to artifacts, and provide little information about the distribution of activities within the library.29 Screening, while lower in throughput, guarantees that every variant is tested and provides quantitative data on its performance.27
Plate-Based and Colony Screening Platforms
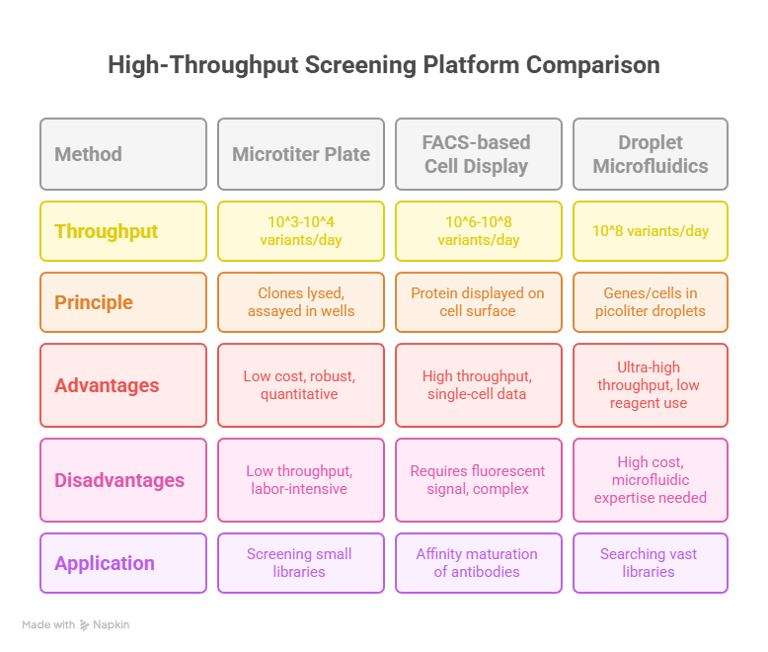
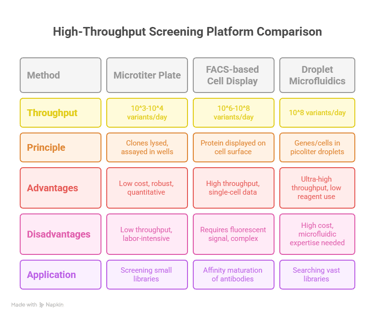
The most traditional screening formats utilize agar plates or multi-well microtiter plates.28 In a colony-based screen, host cells (e.g., bacteria) expressing the enzyme library are grown on a solid medium containing a substrate that produces a visible product.32 For example, in the landmark evolution of subtilisin, colonies expressing active variants formed clear halos on milk-agar plates due to the degradation of the protein casein.33 In a microtiter plate format (typically 96- or 384-well), individual clones are cultured, and their cell lysates are assayed for activity using colorimetric or fluorometric substrates that can be read by a plate reader.29 While these methods are robust and relatively simple to establish, their throughput is limited, typically to 10e3−10e4 variants per day, even with the aid of laboratory automation and robotics.29
Cell-Surface Display Technologies and Fluorescence-Activated Cell Sorting (FACS)
A major advance in throughput was achieved with the development of cell-surface display technologies.30 In these systems, the protein variant is physically tethered to the outer surface of the cell or phage particle that contains its encoding gene.27 Common platforms include yeast surface display and phage display.30 This physical linkage of genotype and phenotype allows the entire cell to be treated as a single unit for screening.30 The library of cells displaying the protein variants can be incubated with a fluorescently labeled target molecule (for binding) or a substrate that generates a fluorescent product that can be captured on the cell surface.30
Cells displaying variants with improved properties will exhibit higher fluorescence intensity.30 These highly fluorescent cells can then be physically isolated from the rest of the population using
Fluorescence-Activated Cell Sorting (FACS).27 A FACS instrument can analyze and sort individual cells at rates of tens of thousands per second, enabling the screening of libraries containing 10e6−10e8 members in a single experiment.27 This dramatic increase in throughput allows for the exploration of much larger and more complex libraries than is feasible with plate-based methods.30
Ultra-High-Throughput Platforms: Droplet-Based Microfluidics
The current frontier of screening technology is represented by ultra-high-throughput (uHTS) platforms, particularly droplet-based microfluidics.34 This technology creates picoliter-volume water-in-oil emulsion droplets, each serving as an independent, miniaturized bioreactor.34 A single gene from the library, along with cell-free expression reagents or a single host cell, is encapsulated within each droplet.34 The expressed enzyme then reacts with a co-encapsulated substrate to produce a fluorescent product, which is contained within the droplet.34 The fluorescence of each droplet is directly proportional to the activity of the enzyme variant it contains.34
These droplets can be generated and screened at rates of thousands per second using a microfluidic sorting device, which functions similarly to a cell sorter.34 This platform has revolutionized the scale of directed evolution experiments, making it possible to screen libraries of
108 variants in just 10 hours, a 1,000-fold increase in speed over robotic systems.34 Furthermore, because the reaction volumes are so small, the total reagent consumption and associated costs are reduced by a million-fold or more, making previously infeasible large-scale screens economically viable.34
The evolution of these screening technologies demonstrates a clear trend: as library generation methods became capable of producing greater diversity, screening platforms had to evolve in parallel to handle the increased scale.35 The development of DNA shuffling and family shuffling created libraries too vast for plate-based methods to search effectively, driving the adoption of FACS.30 In turn, the immense potential of uHTS microfluidics now provides the statistical power necessary to find extremely rare solutions within libraries of hundreds of millions of variants, opening the door to solving even more challenging protein engineering problems.34 For R&D planning, this means the choice of screening platform is intrinsically linked to the library generation strategy; a commitment to a uHTS platform justifies investment in more complex library creation, as it provides the necessary power to find the desired outcome.34
III. Engineering for Robustness: Enhancing Protein Stability
Beyond catalytic performance, a protein's utility in industrial or therapeutic settings is often dictated by its physical robustness.38 The ability to withstand harsh process conditions, maintain activity during long-term storage, and tolerate further engineering is paramount.11 Directed evolution has proven to be an exceptionally powerful tool for enhancing protein stability, delivering biocatalysts and therapeutics that are fit for real-world applications.14
A. The Value Proposition of Stability
For industrial biocatalysis, enhanced stability directly translates into economic value.11 Enzymes that are more resistant to high temperatures, extreme pH, or the presence of organic solvents can be integrated into a wider range of chemical processes, often leading to longer operational lifetimes and reduced catalyst replacement costs.10 In the pharmaceutical arena, a protein therapeutic with greater stability exhibits a longer serum half-life, requiring less frequent dosing, and a longer shelf-life, simplifying storage and distribution logistics.39
Perhaps most critically from an engineering perspective, a stable protein scaffold provides a superior foundation for further evolution.15 A more stable protein is more "evolvable"—it can better tolerate the potentially destabilizing effects of mutations that are introduced to improve or alter its function.15 This tolerance is essential for multi-round campaigns aimed at achieving significant functional leaps, as it prevents the protein from succumbing to misfolding and aggregation as beneficial but structurally disruptive mutations accumulate.15 This makes stability engineering not just an end goal in itself, but often a crucial prerequisite for successful functional engineering.14
B. The Activity-Stability Trade-off
A fundamental principle in protein engineering is the trade-off between activity and stability.15 Natural proteins are typically only marginally stable under their physiological conditions, meaning they have a small free energy of stabilization.15 Mutations introduced to enhance catalytic activity often increase the flexibility of the active site or disrupt existing structural interactions to accommodate a new substrate or transition state.15 While beneficial for function, these changes frequently come at the cost of thermodynamic stability, pushing the protein closer to its unfolding threshold.15
This trade-off represents a common bottleneck in directed evolution.15 As multiple activity-enhancing but destabilizing mutations are accumulated over several rounds, the protein can become so unstable that it fails to fold correctly, leading to a loss of function and an evolutionary dead end.15 Navigating this challenge requires a balanced approach. Successful evolution often depends on the identification of
compensatory stabilizing mutations.4 These are mutations that may be functionally neutral or even slightly deleterious on their own but act to restore stability to the protein scaffold.15 By incorporating these mutations, the protein regains its tolerance for further destabilizing but functionally desirable mutations, allowing the evolutionary process to continue toward higher peaks on the fitness landscape.15
C. Methodologies for Evolving Stability
Screening for improved stability involves subjecting the library of protein variants to a denaturing stress and then identifying those that retain their structure or function.38 The nature of the stress is chosen to mimic the target operational environment.38
Screening for Thermostability
Improving resistance to high temperatures is a common goal.40 Several high-throughput methods have been developed for this purpose:
Interleaved Heat Challenge: This is a direct and effective functional screen.39 A library of variants, typically expressed in microtiter plates, is subjected to a heat challenge at a specific temperature for a defined period.39 The plates are then cooled, and the remaining catalytic activity of each variant is measured.39 Clones that retain a higher fraction of their initial activity are identified as more thermostable.14 In subsequent rounds of evolution, the stringency of the screen can be increased by raising the temperature or extending the duration of the heat challenge.33
Thermal Shift Assays (TSA) / Differential Scanning Fluorimetry (DSF): This biophysical method measures a protein's melting temperature (Tm), the temperature at which 50% of the protein is unfolded.41 The assay is performed in a real-time PCR instrument and uses a fluorescent dye (e.g., SYPRO Orange) that binds to the hydrophobic core of a protein as it unfolds, causing an increase in fluorescence.41 A variant with a higher Tm is more thermostable.41 This method is readily adaptable to 96- or 384-well formats, making it a high-throughput approach for screening purified proteins or cell lysates.41
Protease Susceptibility Assays: This method leverages the principle that well-folded, stable proteins are more resistant to degradation by proteases than less stable or partially unfolded proteins.42 Variants can be incubated with a protease, and stability is inferred by quantifying the amount of intact protein remaining.42 This approach has been cleverly adapted for ultra-high-throughput screening using yeast surface display, where the protein of interest is expressed with a terminal tag.42 Cleavage of the tag by a protease is detected by a loss of fluorescence and can be used to sort a library for more protease-resistant (and thus more stable) variants via FACS.42
Screening for Solvent Tolerance
To evolve enzymes that function in non-aqueous environments, such as in the presence of organic solvents, the screening strategy is analogous to thermal challenges.33 The activity assay is simply performed in the presence of the target solvent.33 A critical consideration is that the selection pressure must be applied gradually.33 Subjecting a library to a very high solvent concentration in the first round would likely denature all variants.33 Instead, a stepwise approach is used, where the solvent concentration is incrementally increased over successive rounds of evolution, allowing the protein population to gradually adapt to the increasingly harsh environment.33
It is crucial for R&D leaders to recognize that "stability" is not a monolithic property.40 The screening conditions must be designed to select for the specific type of stability required by the final application.38 An enzyme evolved for high thermostability via a Tm screen may not necessarily be stable during long-term storage in a liquid formulation at room temperature.40 Similarly, kinetic stability (resistance to irreversible aggregation over time, often measured asT50) is a different physical property from thermodynamic stability (Tm) and may be conferred by different sets of mutations.40 Therefore, aligning the screening assay with the target product profile is essential for success.38
D. Case Study Analysis: Landmark Stability Improvements
The power of these methods is best illustrated by landmark experiments that have yielded enzymes with dramatically enhanced robustness.
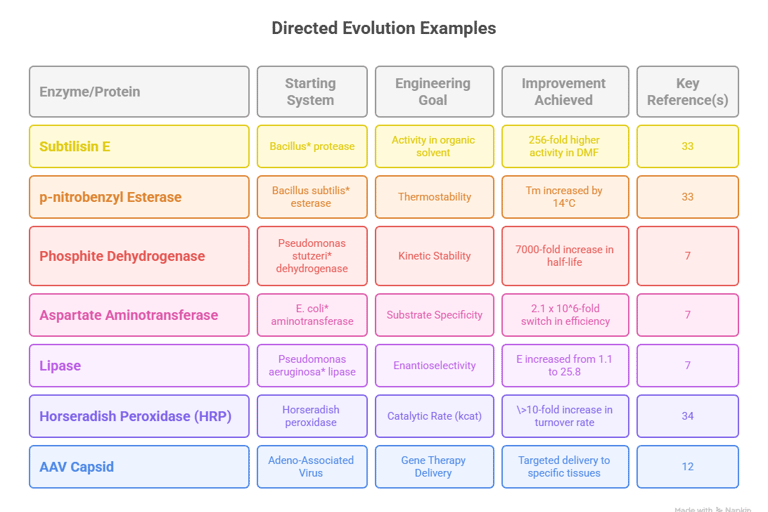
Subtilisin E in Organic Solvent: The seminal 1993 study by Frances Arnold is a benchmark in the field.33 Starting with the protease subtilisin E, four rounds of random mutagenesis and screening on milk-agar plates containing the organic solvent dimethylformamide (DMF) were performed.33 The concentration of DMF was increased in each round, applying progressively stronger selection pressure.33 The final evolved variant contained a combination of ten mutations and exhibited 256-fold higher catalytic activity in 60% (v/v) DMF than the wild-type enzyme.33 This work was a proof-of-principle that directed evolution could adapt enzymes to function in highly unnatural and denaturing environments.33
Thermostabilization of p-nitrobenzyl Esterase: In a demonstration of co-selecting for stability and activity, a p-nitrobenzyl esterase from Bacillus subtilis was subjected to six generations of epPCR and DNA shuffling.33 Screening was performed using an interleaved heat treatment followed by an activity assay.33 The resulting variant showed an increase in its melting temperature (
Tm) of over 14°C, a significant enhancement in thermostability, without compromising its catalytic activity at lower, ambient temperatures.33 This showed that stability could be gained without the common trade-off of reduced low-temperature activity if both properties are constrained during selection.33Phosphite Dehydrogenase for Industrial Use: The wild-type phosphite dehydrogenase from Pseudomonas stutzeri was too unstable for practical use in industrial processes for regenerating NAD(P)H cofactors.7 Through four rounds of epPCR and screening, a variant with 12 mutations was isolated.7 This evolved enzyme displayed a remarkable 7000-fold increase in its half-life at 45°C, transforming it from a fragile laboratory curiosity into a robust biocatalyst suitable for industrial application.7
IV. Engineering for Performance: Improving Protein Function
While stability provides the necessary robustness, the core value of many proteins, particularly enzymes, lies in their function.7 Directed evolution is a premier technology for enhancing catalytic performance, altering substrate specificity to create novel biocatalysts, and fine-tuning selectivity for the precise synthesis of valuable chemicals.7
A. Defining Functional Improvement
The goals of functional engineering are diverse and are defined by the specific application.7 Common targets include:
Catalytic Efficiency (kcat/Km): This is the canonical measure of an enzyme's performance, reflecting both its maximum turnover rate (kcat) and its affinity for its substrate (approximated by Km).3 Directed evolution campaigns can aim to increase
kcat, decrease Km, or, ideally, improve both simultaneously to yield a more efficient catalyst.7Substrate Specificity: Many industrial processes require enzymes that can act on non-natural substrates for which no native enzyme exists.7 Directed evolution is exceptionally well-suited for altering an enzyme's active site to accept a new substrate, often one that is structurally related to the native one.7 This expands the toolbox of biocatalysis for novel chemical synthesis.1
Stereoselectivity and Enantioselectivity: The synthesis of chiral molecules is of paramount importance in the pharmaceutical industry, as different stereoisomers of a drug can have vastly different biological effects.7 Directed evolution can be used to engineer an enzyme to selectively produce one desired stereoisomer (the enantiomer or diastereomer) with high purity, a task that is often challenging for traditional chemical catalysts.7
B. Methodologies for Evolving Function
Screening for function requires an assay that can detect either the consumption of the substrate or the formation of the product in a high-throughput manner.27
Colorimetric and Fluorometric Assays: These are the workhorses of functional screening.29 They rely on a substrate that, upon conversion by the enzyme, produces a product that is either colored or fluorescent.29 The intensity of the signal, which is proportional to the enzyme's activity, can be rapidly quantified in microtiter plates or used as a basis for sorting in FACS.29 A significant challenge arises when the natural substrate of interest does not yield a convenient optical signal.46 In these cases, researchers often resort to using a "proxy" or "surrogate" substrate that is structurally similar to the target but contains a chromogenic or fluorogenic reporter group.46 However, this introduces the risk of evolving an enzyme that is highly active on the proxy but has poor activity on the actual target substrate—a critical consideration for project planning.33
Growth-Coupled Selection Systems: These are elegant and powerful selection strategies that link enzyme activity directly to cell survival.31 For instance, if an enzyme can detoxify an antibiotic, host cells carrying more active variants will be able to survive and grow at higher concentrations of that antibiotic.47 Alternatively, if an enzyme synthesizes a metabolite that is essential for growth (e.g., an amino acid), a host strain that cannot produce this metabolite on its own can be used.31 Only cells carrying enzyme variants active enough to produce the required amount of the metabolite will be able to grow on a minimal medium.31 These systems enable the screening of very large libraries but require clever biological engineering to establish the link between activity and survival.31
Advanced Detection Methods: To overcome the limitations of surrogate substrates, more direct detection methods are being adapted for high-throughput screening.48 High-Throughput Mass Spectrometry (MS) is a particularly promising technology.48 By directly measuring the mass-to-charge ratio of the molecules in a sample, MS can unambiguously identify and quantify the formation of the desired product without the need for any labels or reporter groups.48 The integration of robotic liquid handling systems with fast MS instruments is making this a viable, albeit more complex, screening platform that significantly de-risks a project by ensuring selection for the true target reaction.48
C. Case Study Analysis: Novel Activities and Enhanced Catalysis
Directed evolution has a rich history of successfully re-engineering enzyme function for demanding applications.7
Altering Substrate Specificity: In a remarkable demonstration of functional redesign, an E. coli aspartate aminotransferase, which naturally acts on aspartate, was evolved to prefer valine, a non-native substrate.7 After accumulating 17 mutations—many of which were distal to the active site—the evolved enzyme exhibited a 2.1 x10e6-fold switch in its catalytic efficiency (kcat/Km) in favor of valine.7 This case highlights the profound functional shifts that are possible through directed evolution and underscores the importance of mutations outside the immediate active site in remodeling enzyme function.7
Increasing Enantioselectivity for Chiral Synthesis: The lipase from Pseudomonas aeruginosa was one of the first enzymes to be engineered for improved enantioselectivity.7 Using a combination of epPCR and saturation mutagenesis, its ability to selectively hydrolyze one enantiomer of a chiral ester was dramatically improved.7 The enantiomeric ratio (E value), a measure of selectivity, was increased from 1.1 (indicating almost no selectivity) to 25.8 (indicating high selectivity for one enantiomer).7 This pioneering work demonstrated the utility of directed evolution for creating valuable biocatalysts for the pharmaceutical industry.45
Enhancing Catalytic Rate to the Physical Limit: Horseradish peroxidase (HRP) is already a very efficient natural enzyme.34 Pushing its performance further presented a significant challenge. By using the ultra-high-throughput capacity of droplet microfluidics to screen approximately 10e8 variants, researchers were able to identify HRP mutants with catalytic rates more than 10-fold faster than the parent enzyme.34 The efficiency of the best variants approached the physical limit of catalysis, where the reaction rate is limited only by the speed at which the substrate can diffuse to the enzyme's active site.34 This achievement would have been statistically impossible with lower-throughput screening methods and showcases how uHTS platforms can unlock new levels of performance.34
Creating Novel Catalytic Activity: In an ambitious project combining rational design and directed evolution, researchers successfully installed β-lactamase activity into the protein scaffold of glyoxalase II, an enzyme with no native ability to hydrolyze β-lactam antibiotics.7 The project involved rationally deleting a substrate-binding domain, inserting new loops designed by studying natural β-lactamases, and then using directed evolution to optimize this nascent and very weak activity.7 This demonstrates how directed evolution can build upon a rationally designed starting point to create entirely new functions.7
The recurring theme in these functional evolution studies is the discovery of non-intuitive solutions.9 The beneficial mutations are often not those that an engineer would have predicted based on a static structural model.9 They frequently occur far from the active site and likely function by subtly altering the protein's dynamics, solvation, or overall conformation to better stabilize the transition state of the target reaction.7 This ability to find complex, synergistic, and unexpected solutions is the core value proposition of directed evolution, establishing it as a tool for genuine discovery, not just simple optimization.9.
V. Strategic Implementation: Directed Evolution in the R&D Pipeline
For an R&D organization, adopting directed evolution is not merely a technical choice but a strategic one that influences project timelines, resource allocation, and the very nature of the discovery process.13 Understanding its relationship with other protein engineering strategies, as well as its inherent challenges, is critical for successful implementation.13
A. A Comparative Analysis: Directed Evolution vs. Rational Design
Protein engineering has historically been dominated by two distinct philosophies: rational design and directed evolution.5 Choosing the appropriate strategy depends on the specific problem, the available knowledge, and the desired outcome.9
Rational Design can be compared to the work of an architect.9 It is a knowledge-driven approach that relies on a detailed understanding of a protein's three-dimensional structure and its mechanism of action.5 Using this information, scientists make specific, targeted changes to the amino acid sequence—via site-directed mutagenesis—with the goal of achieving a predictable outcome.5 When structural data is available and the desired change is well-defined (e.g., disrupting a single hydrogen bond), rational design can be precise, fast, and resource-efficient.5 However, its greatest weakness is the profound complexity of proteins.5 Our ability to accurately predict the functional consequences of even single mutations is limited, and unexpected allosteric effects often lead to failed designs.3
Directed Evolution, in contrast, is analogous to the work of a breeder.9 It is a discovery-driven, "black box" approach that mimics natural selection.3 Its signal advantage is that it requires no prior structural or mechanistic information.3 By generating and screening vast numbers of random variants, it can uncover complex, synergistic mutations that lead to dramatic improvements—solutions that are often completely non-intuitive and would never have been predicted by rational approaches.9 The primary limitations of directed evolution are its reliance on a robust, high-throughput screening assay, which can be a significant development hurdle, and the fact that the iterative process can be time-consuming and labor-intensive.5
B. The Hybrid Advantage: Semi-Rational Design and "Smart" Libraries
The most effective modern strategies often occupy the middle ground between these two extremes, in an approach known as semi-rational design or the creation of "smart" libraries.23 This hybrid strategy leverages all available information—from structural models, sequence alignments of homologous proteins, or data from previous evolution rounds—to focus the power of random mutagenesis on specific "hotspot" regions of the protein that are most likely to influence the desired property.23
For example, instead of randomly mutating the entire gene, an engineer might use saturation mutagenesis to explore all possible amino acids at just a handful of residues lining the active site pocket.26 This approach creates libraries that are much smaller than those from random mutagenesis but have a significantly higher density of functional variants.23 This dramatically increases the efficiency of the evolutionary search, often allowing for the identification of improved variants with much lower screening effort and potentially obviating the need for complex ultra-high-throughput platforms.23 This informed approach represents a strategic shift from pure "brute force" screening toward a more efficient, hypothesis-driven exploration of sequence space.23
C. Challenges and Limitations: A Realistic Assessment
Despite its power, directed evolution is not a panacea.5 R&D leaders must be aware of its significant challenges to properly scope projects and manage expectations.5
The Screening Bottleneck: This is the single greatest hurdle in nearly every directed evolution campaign.27 Developing a screening assay that is sensitive, robust, reproducible, scalable to high throughput, and accurately reflects the desired final application property is a major R&D project in itself.27 The lack of a suitable assay is the most common reason a directed evolution project fails or is never initiated.29
The Immensity of Sequence Space: The total number of possible sequences for a typical protein is astronomically large (e.g., 10130 for a 100-amino acid protein).50 Even the largest libraries screened by uHTS platforms (
>108 variants) represent an infinitesimally small fraction of this space.34 The search is, therefore, never exhaustive, and there is no guarantee that the globally optimal sequence will be found.50 The process finds "better" solutions, not necessarily the "best" possible solution.51Evolutionary Dead Ends and Epistasis: Directed evolution is typically a "greedy" algorithm—it selects the best performers from the current generation to parent the next.4 This approach can lead the evolutionary trajectory to a local peak on the fitness landscape, from which it cannot escape to reach a higher, global peak because all adjacent single mutations are deleterious.4 Furthermore, the effects of mutations can be epistatic, meaning the benefit of one mutation depends on the presence of another.13 A mutation that is deleterious on its own might be highly beneficial in combination with a second mutation.13 Such complex pathways involving an initial fitness cost are often missed by standard directed evolution protocols, which purge any variant that is not immediately better than the parent.4
The most effective R&D organizations recognize these challenges and adapt their strategies accordingly.13 They invest not only in wet-lab automation for screening but also in computational biology and data science expertise.13 This allows them to pursue the more efficient semi-rational strategies, using computational models to design smarter libraries that are more likely to yield success.23 Furthermore, they treat every directed evolution campaign, successful or not, as a valuable data generation exercise.52 By capturing and analyzing the full spectrum of mutational effects—the good, the bad, and the neutral—they build proprietary datasets that can be used to train machine learning models.52 These models, in turn, can provide deeper insights into the protein's fitness landscape and guide the design of subsequent, more effective evolutionary campaigns, creating a powerful, data-driven virtuous cycle of innovation.53
References
Methods for the directed evolution of proteins - PubMed, accessed June 30, 2025, https://pubmed.ncbi.nlm.nih.gov/26055155/
Directed Evolution: Methodologies and Applications | Chemical ..., accessed June 30, 2025, https://pubs.acs.org/doi/10.1021/acs.chemrev.1c00260
A primer to directed evolution: current methodologies and future directions - PMC, accessed June 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10074555/
In the light of directed evolution: Pathways of adaptive protein evolution - PNAS, accessed June 30, 2025, https://www.pnas.org/doi/10.1073/pnas.0901522106
Rational design of enzyme activity and enantioselectivity - Frontiers, accessed June 30, 2025, https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2023.1129149/full
Using continuous directed evolution to improve enzymes for plant applications - Oxford Academic, accessed June 30, 2025, https://academic.oup.com/plphys/article/188/2/971/6412949
Directed Evolution: Novel and Improved Enzymes - Zhao Group ..., accessed June 30, 2025, https://zhaogroup.chbe.illinois.edu/publications/HZ61.pdf
Directed Evolution: Past, Present and Future - PMC, accessed June 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4344831/
Protein Engineering Showdown: Rational Design vs Directed Evolution - Patsnap Synapse, accessed June 30, 2025, https://synapse.patsnap.com/article/protein-engineering-showdown-rational-design-vs-directed-evolution
Directed evolution of industrial enzymes: an update - PubMed, accessed June 30, 2025, https://pubmed.ncbi.nlm.nih.gov/12943855/
Directed evolution of industrial enzymes: An update | Request PDF - ResearchGate, accessed June 30, 2025, https://www.researchgate.net/publication/10592998_Directed_evolution_of_industrial_enzymes_An_update
Synthetic Biology, Directed Evolution, and the Rational Design of New Cardiovascular Therapeutics: Are We There Yet? - PMC, accessed June 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10401280/
Synthetic biology for the directed evolution of protein biocatalysts: navigating sequence space intelligently - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C4CS00351A, accessed June 30, 2025, https://pubs.rsc.org/en/content/articlehtml/2015/cs/c4cs00351a
Directed Evolution of New and Improved Enzyme Functions Using an Evolutionary Intermediate and Multidirectional Search - ACS Publications, accessed June 30, 2025, https://pubs.acs.org/doi/10.1021/cb500809f
Directed evolution methods for overcoming trade-offs between protein activity and stability, accessed June 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7384606/
Advances in the directed evolution of proteins - College of Biological Sciences, accessed June 30, 2025, https://cbs.umn.edu/sites/cbs.umn.edu/files/migrated-files/downloads/Lane-Seelig.DirectedEvolutionOfNovelProteins.pdf
pmc.ncbi.nlm.nih.gov, accessed June 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC390300/#:~:text=These%20methods%2C%20which%20include%20the,within%20the%20DNA%20being%20copied.
A primer to directed evolution: current methodologies and future directions - RSC Publishing, accessed June 30, 2025, https://pubs.rsc.org/en/content/articlehtml/2023/cb/d2cb00231k
Error-prone PCR, accessed June 30, 2025, https://sites.ffclrp.usp.br/pbbg/english/topics/molecular_biology/error-prone_pcr.htm
(PDF) Mutant Library Construction in Directed Molecular Evolution: Casting a Wider Net, accessed June 30, 2025, https://www.researchgate.net/publication/6844467_Mutant_Library_Construction_in_Directed_Molecular_Evolution_Casting_a_Wider_Net
DNA shuffling – Knowledge and References - Taylor & Francis, accessed June 30, 2025, https://taylorandfrancis.com/knowledge/Engineering_and_technology/Chemical_engineering/DNA_shuffling/
Anticipatory evolution and DNA shuffling - PMC - PubMed Central, accessed June 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC139397/
Beyond directed evolution - semi-rational protein engineering and design - PMC, accessed June 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC2982887/
Assessing directed evolution methods for the generation of biosynthetic enzymes with potential in drug biosynthesis - PMC, accessed June 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3155183/
Site-directed mutant libraries for isolating minimal mutations yielding functional changes | Protein Engineering, Design and Selection | Oxford Academic, accessed June 30, 2025, https://academic.oup.com/peds/article/30/5/347/3064206
Directed Enzyme Evolution and High-Throughput Screening - Zhao Group @ UIUC, accessed June 30, 2025, https://zhaogroup.chbe.illinois.edu/publications/HZ72.pdf
High Throughput Screening and Selection Methods for Directed ..., accessed June 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4461044/
Advances in ultrahigh-throughput screening for directed enzyme evolution - Washington State University, accessed June 30, 2025, https://searchit.libraries.wsu.edu/discovery/fulldisplay/cdi_proquest_journals_2331799739/01ALLIANCE_WSU:WSU
High Throughput Screening and Selection Methods for Directed Enzyme Evolution | Industrial & Engineering Chemistry Research - ACS Publications, accessed June 30, 2025, https://pubs.acs.org/doi/10.1021/ie503060a
High-throughput directed evolution: a golden era for protein science, accessed June 30, 2025, https://eprints.whiterose.ac.uk/id/eprint/183791/7/PIIS2589597422000648.pdf
A growth-based screening strategy for engineering the catalytic activity of an oxygen-sensitive formate dehydrogenase | Applied and Environmental Microbiology - ASM Journals, accessed June 30, 2025, https://journals.asm.org/doi/abs/10.1128/aem.01472-24
(PDF) Directed Evolution and Solid Phase Enzyme Screening - ResearchGate, accessed June 30, 2025, https://www.researchgate.net/publication/374227473_Directed_Evolution_and_Solid_Phase_Enzyme_Screening
DIRECTED EVOLUTION OF ENZYMES AND BINDING PROTEINS - Nobel Prize, accessed June 30, 2025, https://www.nobelprize.org/uploads/2018/10/advanced-chemistryprize-2018.pdf
Ultrahigh-throughput screening in drop-based microfluidics for directed evolution | PNAS, accessed June 30, 2025, https://www.pnas.org/doi/10.1073/pnas.0910781107
High-Throughput Screening in Protein Engineering: Recent Advances and Future Perspectives - PubMed, accessed June 30, 2025, https://pubmed.ncbi.nlm.nih.gov/26492240/
Directed Evolution in Drops: Molecular Aspects and Applications | ACS Synthetic Biology, accessed June 30, 2025, https://pubs.acs.org/doi/10.1021/acssynbio.1c00313
Ultrahigh-Throughput Enzyme Engineering and Discovery in In Vitro Compartments | Chemical Reviews - ACS Publications, accessed June 30, 2025, https://pubs.acs.org/doi/10.1021/acs.chemrev.2c00910
Directed evolution of enzyme stability | Request PDF - ResearchGate, accessed June 30, 2025, https://www.researchgate.net/publication/7879881_Directed_evolution_of_enzyme_stability
Directed evolution of an extremely stable fluorescent protein - Oxford Academic, accessed June 30, 2025, https://academic.oup.com/peds/article/22/5/313/1499205
Evaluating Protein Engineering Thermostability Prediction Tools Using an Independently Generated Dataset | ACS Omega - ACS Publications, accessed June 30, 2025, https://pubs.acs.org/doi/10.1021/acsomega.9b04105
Protein Stability Using Thermal Shift Assay (TSA): pH Tolerance - ResearchGate, accessed June 30, 2025, https://www.researchgate.net/publication/354624395_Protein_Stability_Using_Thermal_Shift_Assay_TSA_pH_Tolerance
High-throughput developability assays enable library-scale identification of producible protein scaffold variants | PNAS, accessed June 30, 2025, https://www.pnas.org/doi/10.1073/pnas.2026658118
Frances Arnold - Wikipedia, accessed June 30, 2025, https://en.wikipedia.org/wiki/Frances_Arnold
Directed enzyme evolution: climbing fitness peaks one amino acid at a time - PMC, accessed June 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC2703427/
Directed Evolution of Enzymes as Catalysts in Synthetic Organic Chemistry Manfred T. Reetz - MPI für Kohlenforschung, accessed June 30, 2025, https://www.kofo.mpg.de/258958/Directed-Evolution-of-Enzymes.pdf
Enlightening the Path to Protein Engineering: Chemoselective Turn-On Probes for High-Throughput Screening of Enzymatic Activity | Chemical Reviews - ACS Publications, accessed June 30, 2025, https://pubs.acs.org/doi/10.1021/acs.chemrev.2c00304
Directed evolution of industrial enzymes - Chemistry and Chemical Engineering Division, accessed June 30, 2025, https://cheme.caltech.edu/groups/fha/publications/Schmidt-Dannert+Arnold_TrendsBiotech_1999.pdf
Directed evolution of a cyclodipeptide synthase with new activities via label-free mass spectrometric screening - RSC Publishing, accessed June 30, 2025, https://pubs.rsc.org/en/content/articlelanding/2022/sc/d2sc01637k
“Multi-Agent” Screening Improves the Efficiency of Directed Enzyme Evolution | bioRxiv, accessed June 30, 2025, https://www.biorxiv.org/content/10.1101/2021.04.06.438652v1
Directed evolution - Wikipedia, accessed June 30, 2025, https://en.wikipedia.org/wiki/Directed_evolution
Opportunities and Challenges for Machine Learning-Assisted Enzyme Engineering | ACS Central Science - ACS Publications, accessed June 30, 2025, https://pubs.acs.org/doi/10.1021/acscentsci.3c01275
Summary of main challenges and future developments of directed evolution. - ResearchGate, accessed June 30, 2025, https://www.researchgate.net/figure/Summary-of-main-challenges-and-future-developments-of-directed-evolution_fig4_367506757
Machine learning-assisted directed protein evolution with combinatorial libraries - PNAS, accessed June 30, 2025, https://www.pnas.org/doi/10.1073/pnas.1901979116
Directed Evolution: Bringing New Chemistry to Life - PMC, accessed June 30, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC5901037/
Directed Evolution Methods for Enzyme Engineering - MDPI, accessed June 30, 2025, https://www.mdpi.com/1420-3049/26/18/5599
Advances in ultrahigh-throughput screening technologies for protein evolution | Request PDF - ResearchGate, accessed June 30, 2025, https://www.researchgate.net/publication/370193821_Advances_in_ultrahigh-throughput_screening_technologies_for_protein_evolution
The Nobel Prize in Chemistry 2018 - Popular information - NobelPrize.org, accessed June 30, 2025, https://www.nobelprize.org/prizes/chemistry/2018/popular-information/